Ice Table For Diprotic Acid . Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to find the ph given a concentration of a weak. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. An acid that contains more than one ionizable proton is a polyprotic acid. The protons of these acids ionize in steps. Using provided information, an ice table for this first step is prepared: Substituting the equilibrium concentrations into the equilibrium equation. Notice that with the first ionization reaction,. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. 117k views 8 years ago chemical. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid.
from www.chegg.com
Using provided information, an ice table for this first step is prepared: 117k views 8 years ago chemical. Notice that with the first ionization reaction,. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. Substituting the equilibrium concentrations into the equilibrium equation. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the.
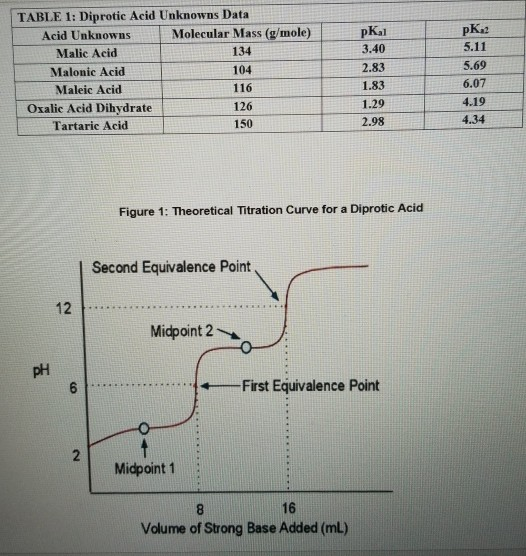
Solved TABLE 1 Diprotic Acid Unknowns Data Acid Unknowns
Ice Table For Diprotic Acid Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to find the ph given a concentration of a weak. An acid that contains more than one ionizable proton is a polyprotic acid. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. 117k views 8 years ago chemical. Substituting the equilibrium concentrations into the equilibrium equation. The protons of these acids ionize in steps. Notice that with the first ionization reaction,. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. Using provided information, an ice table for this first step is prepared: This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to find the ph given a concentration of a weak.
From www.studocu.com
Lecture 5 Notes Lecture 5 Equilibrium and ICE Tables Equilibrium Ice Table For Diprotic Acid 117k views 8 years ago chemical. An acid that contains more than one ionizable proton is a polyprotic acid. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. The. Ice Table For Diprotic Acid.
From www.youtube.com
Calculating [OH] and pH using conjugate bases, Kb and ICE Tables YouTube Ice Table For Diprotic Acid The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. Using provided information, an ice table for this first step is prepared: Not to get into too much detail between monoprotic and polyprotic. Ice Table For Diprotic Acid.
From www.youtube.com
Ka, Kb, pH, pOH in ICE tables YouTube Ice Table For Diprotic Acid Using provided information, an ice table for this first step is prepared: The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. The ice tables below display both ionization reactions, starting. Ice Table For Diprotic Acid.
From ar.inspiredpencil.com
Acids And Bases List Ice Table For Diprotic Acid Notice that with the first ionization reaction,. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Substituting the equilibrium concentrations into the equilibrium equation. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. Not to get into too. Ice Table For Diprotic Acid.
From www.plumpuddingchemistry.com
5 key concepts that make up AP Chemistry Acids and Bases Vaishnavi Ice Table For Diprotic Acid Using provided information, an ice table for this first step is prepared: An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. Notice that with the first ionization reaction,. The protons of these acids ionize in steps. Substituting the equilibrium concentrations into the equilibrium equation. An acid that contains more than one. Ice Table For Diprotic Acid.
From www.chegg.com
Solved Lab Partner SUMMARY DATA TABLE Mass of diprotic acid Ice Table For Diprotic Acid Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. 117k views. Ice Table For Diprotic Acid.
From www.youtube.com
Example Calculate pH for a Weak Acid using an ICE Table YouTube Ice Table For Diprotic Acid This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Substituting the equilibrium concentrations into the equilibrium equation. Notice that with the first ionization reaction,. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. Not to get into too much detail between monoprotic and polyprotic. Ice Table For Diprotic Acid.
From www.youtube.com
General Chemistry III Solving ICETables for Diprotic Systems YouTube Ice Table For Diprotic Acid An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. 117k views 8 years ago chemical. The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to. Ice Table For Diprotic Acid.
From www.numerade.com
SOLVED Without using an ice table, determine the concentration of CO32 Ice Table For Diprotic Acid Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. 117k views 8 years ago chemical. Notice that with the first ionization reaction,. The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. Not to get into. Ice Table For Diprotic Acid.
From www.chegg.com
Solved Determine the Ka for an acid by constructing an ICE Ice Table For Diprotic Acid Using provided information, an ice table for this first step is prepared: Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to find the ph given a concentration of a weak. An acid that contains more than one ionizable proton is a polyprotic acid. Ice tables are composed of the concentrations of molecules. Ice Table For Diprotic Acid.
From www.chegg.com
Solved Titration of a Diprotic Acid Identifying an Unknown A Ice Table For Diprotic Acid This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to find the ph given a concentration of a weak. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and. Ice Table For Diprotic Acid.
From www.chegg.com
Solved Determine the Ka for an acid by constructing an ICE Ice Table For Diprotic Acid Using provided information, an ice table for this first step is prepared: 117k views 8 years ago chemical. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. Substituting the equilibrium concentrations into the equilibrium equation. An acid that contains more than one ionizable proton is a polyprotic acid. The protons of. Ice Table For Diprotic Acid.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps Ice Table For Diprotic Acid Notice that with the first ionization reaction,. 117k views 8 years ago chemical. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. The protons of these acids ionize in steps. Using provided information, an ice. Ice Table For Diprotic Acid.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5123561 Ice Table For Diprotic Acid Substituting the equilibrium concentrations into the equilibrium equation. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. 117k views 8 years ago chemical. Not to get into too much detail between monoprotic and polyprotic acids, but if you desire to find the ph given a. Ice Table For Diprotic Acid.
From www.chegg.com
Solved TABLE 1 Diprotic Acid Unknowns Data Acid Unknowns Ice Table For Diprotic Acid 117k views 8 years ago chemical. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for. Notice that with the first ionization reaction,. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. The ice tables below display both ionization reactions,. Ice Table For Diprotic Acid.
From www.youtube.com
Shortcut to Calculate pH of Weak Acids w/ Examples, Practice Problem Ice Table For Diprotic Acid The protons of these acids ionize in steps. Using provided information, an ice table for this first step is prepared: 117k views 8 years ago chemical. The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. Ice tables are composed of the concentrations of molecules in solution in different stages of. Ice Table For Diprotic Acid.
From www.youtube.com
Polyprotic AcidBase Part 2 Buffers YouTube Ice Table For Diprotic Acid The ice tables below display both ionization reactions, starting with a 10.0 m solution of h 2 co 3. 117k views 8 years ago chemical. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. Notice that with the first ionization reaction,. Substituting the equilibrium concentrations into the equilibrium. Ice Table For Diprotic Acid.
From www.chegg.com
Solved This is the pH curve for titration of a diprotic acid Ice Table For Diprotic Acid Substituting the equilibrium concentrations into the equilibrium equation. Ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the. Using provided information, an ice table for this first step is prepared: An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful. Ice Table For Diprotic Acid.